RGUHS Nat. J. Pub. Heal. Sci Vol: 15 Issue: 4 eISSN: pISSN
Dear Authors,
We invite you to watch this comprehensive video guide on the process of submitting your article online. This video will provide you with step-by-step instructions to ensure a smooth and successful submission.
Thank you for your attention and cooperation.
1Dr. Chaithra S, Department of Community Medicine, Bangalore Medical College and Research Institute, Bengaluru, Karnataka, India.
2Department of Community Medicine, Bangalore Medical College and Research Institute, Bengaluru, Karnataka, India
3Independent Consultant (Vector Borne Diseases), Bengaluru, Karnataka, India
*Corresponding Author:
Dr. Chaithra S, Department of Community Medicine, Bangalore Medical College and Research Institute, Bengaluru, Karnataka, India., Email: chaithra.snp6295@gmail.com
Abstract
Background: Malaria continues to pose a significant health challenge in India, despite ongoing control and elimination initiatives. The disease affects health systems, economies, and communities, particularly in areas with favorable environmental conditions. While surveillance and vector control interventions have significantly lowered malaria incidence at both the state level and in Bangalore Urban, persistent challenges continue to impede complete eradication.
Aim: To evaluate the effectiveness of vector control measures and the availability of essential resources for malaria elimination in Bangalore Urban.
Methods: A retrospective evaluation of vector control measures was conducted in low-coverage areas of Bangalore Urban from January 2018 to December 2022. Data were collected from the Regional Malaria Control Office and the District Vector Borne Disease Control Office. Information was gathered through semi-structured questionnaires and interviews with government health officials. The data were entered into MS Excel and analyzed using SPSS 26.0.
Results: The study found that essential vector control tools, including Long-Lasting Insecticidal Nets (LLINs), adulticides (Temephos 50%), insecticides (DDT 50%, S.P. 10% & 5%, Malathion 95%, Deltamethrin 2.5%) and spray pumps, were available. Various malaria medications, such as Artemisinin-based combination therapy (ACT), Quinine sulphate, Combi Pack (CQ+PQ), Chloroquine phosphate, Primaquine phosphate, Rapid Diagnostic (RD) kits, Artesunate, Artemether, and Quinine injections, were also accessible.
Conclusion: The findings indicate that Bangalore Urban possesses the essential resources and vector control tools required for malaria management. However, continued efforts are necessary to address challenges and improve efficiency. Continuous monitoring, adaptive strategies, and collaborative efforts are critical to strengthening district-level preparedness and achieving sustainable malaria elimination in the region.
Keywords
Downloads
-
1FullTextPDF
Article
Introduction
Malaria remains a significant global and national public health challenge, with an estimated 249 million cases worldwide in 2022.1 India contributed 1.7% of global cases and 1.2% of deaths.2 Despite substantial progress under the National Vector Borne Disease Control Programme (NVBDCP) in reducing malaria incidence, several challenges remain. Approximately 95% of India’s population lives in malaria-endemic areas, and 80% of cases occur in tribal, hilly, and inaccessible regions. Although the Slide Positivity Rate (SPR) has decreased from 3.50 in 1995 to 0.19 in 2020, the proportion of Plasmodium falciparum (Pf) cases has increased from 39% to 63.84%, indicating gaps in surveillance and the need for targeted interventions to meet the Sustainable Development Goal of reducing malaria incidence by 90% by 2030.3,4
Despite progress, significant gaps remain in malaria control strategies that hinder the achievement of elimination targets.5 Data from the NVBDCP and WHO highlight issues such as inadequate surveillance systems, inconsistent use of insecticide-treated nets (ITNs), and incomplete coverage of indoor residual spraying (IRS).6 Additionally, urban areas like Bangalore face unique challenges, including rapid urbanization, high population density, and diverse community practices that affect malaria transmission dynamics.7,8,9,10 There is also a lack of detailed entomological data to guide vector control and insufficient community engagement in prevention efforts.11-13 These gaps indicate the need for a more integrated and comprehensive approach to malaria control that addresses specific urban challenges.
This study aimed to identify gaps in current malaria prevention and control measures and provide evidence-based recommendations for improvement. The research focuses on understanding the strengths and weaknesses of existing efforts to enhance malaria control strategies. The findings will support policymakers and health practitioners in optimizing interventions and resource allocation, strengthening district preparedness for sustainable malaria elimination.
Materials and Methods
Approval for the study was obtained from the Institutional Ethics Committee of Bangalore Medical College and Research Institute (No. BMCRI/EC/20/23-24), and permission was granted by the relevant authorities to conduct the research. Malaria cases confirmed by Rapid Diagnostic Testing (RDT) and/or microscopy were included in the study. Data were collected from government sector sources, including paper-based annual reports and epidemiological records from the Regional Office, utilizing the malaria surveillance toolkit.14 A detailed questionnaire gathered information on vector control measures, such as the types of interventions, their frequency, coverage, resources, logistics, and challenges encountered.
A retrospective evaluation of vector control measures from 2018 to 2022 was conducted in Bangalore Urban to assess the effectiveness of existing interventions and identify gaps. This evaluation involved mapping confirmed malaria cases and conducting vector surveys in areas with low intervention coverage over six months, from December 2023 to May 2024.
Retrospective evaluation of vector control measures was conducted from 2018 to 2022 in Bangalore Urban to assess the effectiveness of existing interventions and identify gaps (Figure 1). This evaluation inv-olved mapping confirmed malaria cases and conducting vector surveys in low-coverage areas over six months (December 2023 to May 2024). Interviews were conducted with key stakeholders, including public health officials, field workers, and community representatives, to gather qualitative insights into vector control practices and community participation.
Data Analysis
Data were collected and entered into Excel, then analyzed using IBM SPSS Statistics for Windows, Version 26.0. Descriptive statistics were applied to summarize the data and identify patterns and trends in vector cont-wrol measures. This analysis provided a comprehensive assessment of the effectiveness of current vector control strategies and highlighted areas requiring improvement.
Results
In Table 1, the malaria-metric indices from 2018 to 2022 reveal a consistently low prevalence of malaria in the examined populations, characterized by fluctuating annual blood examination rates (ABER) ranging from 3.8% to 13.1%. Despite occasional positive cases detected in 2019 and 2020, annual parasite incidence (API) remained zero throughout, reflecting no new reported cases per 1,000 population. No malaria-related deaths were recorded during this period, highlighting effective control measures and low mortality rates associated with the disease.
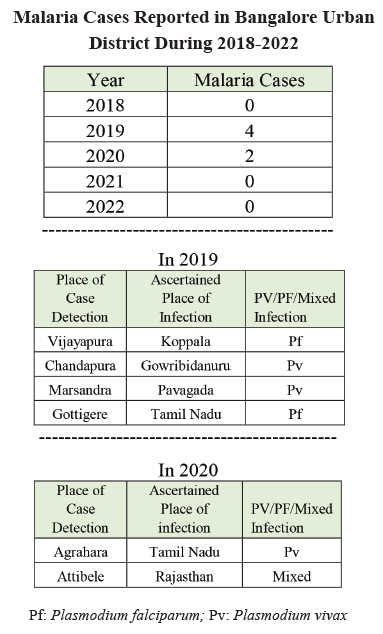
In Table 2, the line listing of malaria cases from 2018 to 2022 shows sporadic imported cases each year, with no indigenous cases reported. Total malaria cases remained low, with 2019 recording the highest count of four cases, including two Plasmodium vivax (Pv) and Plasmodium falciparum (Pf) cases, all categorized as imported. The absence of indigenous cases suggests effective control measures against local transmission during this period. Table 3 data indicate a mixed performance in malaria surveillance and response indicators across the study period in Bangalore Urban. While the number of sub-centres remained consistent and there was a general improvement in the proportion of blood smear examinations comple-ted within 24 to 72 hours from 2018 to 2022, other metrics showed variability. The decrease in sub-centres with an ABER <10 in 2019, followed by an increase in 2022, suggests inconsistent implementation of active surveillance measures. Improvements in surveillance coverage by auxiliary nurse midwives (ANM) and multi-purpose workers (MPW) by 2022 reflect strengthened monitoring efforts. However, the fluctuating ratio of active to passive surveillance and variations in the timeliness of blood smear examinations highlight the need for more consistent surveillance practices.
Table 4 illustrates and emphasizes the available human resources, training, and tests related to quality assurance.
The inventory management data from 2018 to 2022 highlight fluctuations in the availability and utilization of stocks for malaria control measures. Starting with an opening balance of 51.5 in 2018, stocks saw varying increments and decrements annually, with the highest total stock recorded in 2022 at 262.5 units. Utilization rates fluctuated, with peaks in 2019 and 2021, where 77.8 and 60 units were utilized, respectively. Despite these fluctuations, closing balances generally stabilized over the years, suggesting effective management of stock levels to meet operational needs while maintaining reserves for future demands in malaria control efforts.
Table 5 illustrates the financial performance, as well as the availability and utilization of funds.
The data indicate that Accredited Social Health Activist (ASHA) incentives were fully utilized each year, except in 2020-2021, when only 61.05% was used. Monitoring, Evaluation, and Supervision (M, E & S) funds showed consistent utilization; however, both allocation and utilization decreased over time. Epidemic preparedness under the National Anti-Malaria Management Information System (NAMMIS) was well funded and utilized, particularly during 2019-2021, with near-complete utilization in each period.
Entomological Surveillance
The entomological study of vector species from 2018 to 2022 revealed key trends in malaria transmission. In 2018, Anopheles culicifacies and Anopheles stephensi were identified as the primary vectors. However, in 2019, no primary vector was reported, indicating a potential decline in cases. In 2020 and 2021, An. stephensi re-emerged as the primary vector. Meanwhile, An. subpictus consistently served as a secondary vector throughout the study period. This persistence of An. subpictus, along with the fluctuating dominance of An. stephensi, highlights the ongoing challenges in malaria control and the need for continuous vector surveillance.
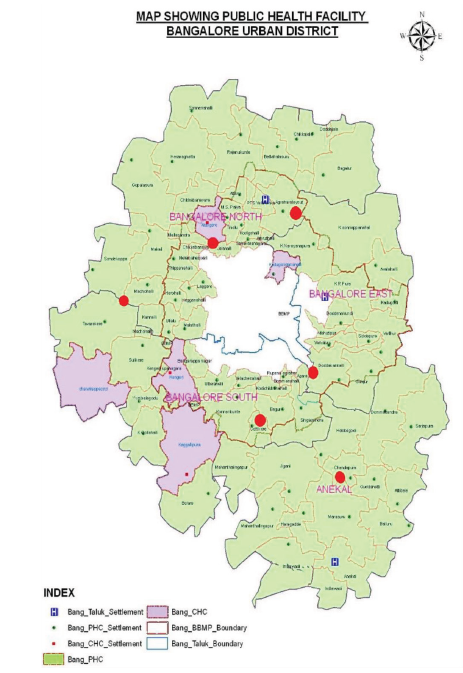
Vulnerable Areas and Breeding Sites in Bangalore Urban District
Vulnerable and receptive areas of Bangalore Urban District include the stone quarry regions located in Madapattana village of Chandrappa circle primary health center (PHC), Bettahalasuru area, and Ragihalli quarry of Mahanthilingapura PHC. Both migrant and local labourers from nearby villages are employed in these quarries. Since majority of the migrant workers originate from malaria-endemic states, there exists a significant risk of re-introduction of malaria, mainly through asymptomatic carriers, given that these areas remain receptive due to the presence of potential vectors. The breeding sources of malaria vectors in Bangalore Urban District include 785 water bodies distributed across different taluks. In Anekal, there are 337 water bodies, of which 153 have been stocked with fishes. East taluk has 171 water bodies, with 50 containing fishes. South taluk includes 86 water bodies, with fishes present in 32 of them, while North taluk has 191 water bodies, of which 104 have been stocked with fishes. Overall, fishes have been released into 339 water bodies across all taluks to control vector breeding.
Anti-malarial drugs available include Chloroquine phos-phate 250 mg (150 mg base), Primaquine phosphate 2.5 mg, Primaquine phosphate 7.5 mg, and a combination pack of Chloroquine and Primaquine (CQ+PQ). Additionally, Artemisinin-based combination therapy (ACT) Combi Blister Packs are available for adults, and for paediatric patients across different age categories: 0-1 year, 1-4 years, 5-8 years, and 9-14 years. For severe cases, Arteether injection and Artesunate injection are administered. Rapid Diagnostic (RD) Kits for Malaria (Bivalent) are also available to facilitate prompt diagnosis.
Insecticides used for mosquito control include DDT 50% WDP, Synthetic Pyrethroid (S.P.) 10% WP, S.P. 5% WP, and Malathion 25% WP. Other insecticides comprise S.P. Liquid/Deltamethrine 2.5% Flow, along with various larvicides/adulticides like Temephos 50% EC, Pyrethrum Extract 2%, Malathion Technical (liquid), and Cyphanothrin 5% EC. Bacillus thuringiensis israel-ensis (Bti) formulations, available in both WP (wettable powder) and AS (aqueous suspension) forms, are also utilized. Bed nets, including Long-Lasting Insecticidal Nets (LLINs) in single, double, and family sizes, are critical for personal protection, along with standard mosquito bed nets.
For vector-borne diseases such as dengue and chikungunya, diagnostic tools available include the NS1 Anti-gen (ELISA-based) test, IgM ELISA kits (Dengue), and IgM ELISA kits for chikungunya and Japanese Encephalitis (JE). Equipment used in vector control includes stirrup pumps, hand compression pumps, mist blowers, fogging machines (portable and vehicle-mounted), as well as blood lancets, micro glass slides, and microscopes (monocular and binocular types). Additionally, Primaquine 15 mg and other essential medical supplies support the fight against vector-borne diseases and suggest a comprehensive readiness to address diverse operational needs and challenges in malaria surveillance and response efforts.
Discussion
The study by Manju Rahi et al., highlights that, despite a substantial reduction in malaria cases, India continues to face challenges, including insufficient real-time surveillance and inadequate monitoring of at-risk populations. For instance, in 2019, India accounted for 88% of malaria cases reported in the WHO South-East Asia Region and nearly half of the global Plasmodium vivax cases in 2018.15 In the current study, both P. falciparum and P. vivax cases were detected. A retrospective case analysis by Krishna C et al., in Tumkur (2017) reported a decline in malaria and a consistent drop in API, with no fatalities or outbreaks recorded over 15 years (2001-2015), with an average ABER of 23.23% in the last three years.16 In the current study, both API and SPR have consistently remained at zero, while ABER increased from 9 in 2018 to 13.1 in 2022, despite no indigenous cases in the past five years (2018-2022). Malaria cases were only observed in 2019 and 2020, with a decline in passive case screening during the COVID-19 pandemic in 2020.
The study by Mishra AK et al., found Anopheles culicifacies (38.5% sibling species C) to be the most prevalent vector. In 2019, it showed resistance to DDT, Malathion, and Alphacypermethrin, with insecticide mortality rates dropping from 82-98% on day one to 35-62% by day 30. An. culicifacies tested positive for Plasmodium falciparum infection using PCR testing.17 Smith Gueye et al. discussed vector control efforts like integrated management, but noted limited impact due to non-specific responses and poor implementation. Malaria resurgence was tied to halted IRS coverage, funding issues, and operational constraints. Bed net usage lacked adequate coverage measurement, and larval control data were insufficient.18 In the current study, despite increased funding for malaria control, adequate training for the effective utilization of resources was lacking.
Gayan Dharmasiri et al., (2017) reported the discovery of Anopheles stephensi in Mannar Island in Northern Sri Lanka, posing a potential threat to malaria prevention efforts despite the country’s malaria-free status. The presence of An. stephensi necessitates ongoing vigilance, funding, and comprehensive preventive measures to prevent malaria reintroduction.19 Similarly, in Bangalore district, entomological surveillance has identified vec-tors like An. culicifacies and An. stephensi, challenging efforts to maintain malaria elimination.
Dhiman R et al., (2010-2013) conducted a study in Uttarakhand, which showed that rising temperatures extended the malaria transmission season, resulting in increased densities of An. culicifacies and An. Fluviati-lis. This underscores the need for tailored preparedness and heightened surveillance to address climate-induced shifts.20 The entomological data also revealed varying vector densities during the monsoon season, due to factors such as percolation of water, availability of temporary settlements, migratory populations, and open drainage systems.
The study by Chalageri VH et al., analyzed district-level malaria data from 1991 to 2021, collected from the Regional Office for Health & Family Welfare (ROH & FW), Bangalore. The data were categorized into three decades, and sequence plots of the moving average of the Annual Parasite Index (API) were created. A generalized estimating equation model with a Poisson distribution was used to assess differences in malaria indicators before and after interventions like LLINs, RDTs with ACT, and the introduction of Guppy and Gambusia fishes.21 Malaria surveillance data from 2018 to 2022 in Bangalore Urban District showed low prevalence, sporadic imported cases, and no indigenous cases, reflecting the effectiveness of control measures. The district's inventory of anti-malarial drugs, vector control tools, and quality assurance systems further demonstrates its robust management of malaria risks.
Limitations
The study's limitations include the difficulties of gath-ering information from all practitioners in Bangalore's urban areas owing to resource limitations. Time constraints led to a reduction in the frequency of visits to PHCs and incomplete surveillance in the private sector. Furthermore, the absence of research articles that address similar study topics and objectives posed a challenge in forming a dependable comparison.
Conclusion
The comprehensive inventory of equipment, anti-malarial drugs, and vector control measures demonstrates a well-rounded approach to malaria control in the study area. The presence of diverse equipment, such as stirrup pumps, fogging machines, and microscopes, highlights preparedness for both vector control and diagnostic activities. This infrastructure is complemented by a range of anti-malarial drugs tailored to different age groups, ensuring accessibility to effective treatment. Such a robust inventory not only supports immediate response capabilities but also reflects a proactive commitment to addressing malaria transmission across all stages - from prevention through vector control to treatment with appropriate medications. The availability of specialized tools, like fogging machines and LLINs, further underscores a targeted approach to reduce mosquito populations and the overall disease burden.
Overall, this integrated approach highlights a concerted effort to enhance public health outcomes by addressing the multifaceted challenges posed by malaria, ensuring readiness to mitigate its impact and promote community well-being effectively. Continued investment in these resources and strategies is crucial for sustaining progress towards malaria elimination goals in the region.
Conflict of interest
None
Financial support
None
Acknowledgement
We are grateful to Dr. K. Ravi Kumar, Independent Consultant (Vector Borne Disease) and Dr. Bhujabali, District Vector Borne Disease Control Officer and all of the field enumerators who assisted with data gathering and to the field technical person in charge. Additionally, we would like to express our gratitude to all provincial and regional governments, and the health offices in the pertinent research areas.
Supporting File
References
1. World Health Organization. Malaria. World Health Organization. 2023. Available from: https://www. who.int/news-room/questions-and-answers/item/ malaria
2. World Health Organization. Summary of World Malaria Report 2021 [Internet]. Geneva: WHO; 2021. Available from: https://www.who.int/india/ health-topics/malaria/summary-of-world-malaria-report-2021
3. National Centre for Vector Borne Diseases Control. National Strategic Plan for Malaria Elimination in India 2023-2027. National Centre for Vector Borne Diseases Control; 2023. Available from: https:// ncvbdc.mohfw.gov.in/Doc/National-Strategic-Plan- Malaria-2023-27.pdf
4. World Health Organization. SDG Target 3.3: Communicable diseases [Internet]. Geneva: WHO; 2023. Available from: https://www.who.int/data/gho/ data/themes/topics/sdg-target-3_3-communicable-diseases
5. Paaijmans KP, Lobo NF. Gaps in protection: the actual challenge in malaria elimination. Malar J 2023;22:46.
6. Pryce J, Medley N, Choi L. Indoor residual spraying for preventing malaria in communities using insecticide-treated nets. Cochrane Database Syst Rev 2022;1(1):CD012688.
7. World Health Organization. Global framework for the response to malaria in urban areas [Internet]. Geneva: WHO; 2021. Available from: https://www. who.int/publications/i/item/9789240061781.
8. Reyes R, Ahn R, Thurber K, et al. Urbanization and infectious diseases: General principles, historical perspectives, and contemporary challenges. Challenges in Infectious Diseases 2012 May 19:123-146.
9. Santos-Vega M, Bouma MJ, Kohli V, et al. Population density, climate variables and poverty synergistically structure spatial risk in urban malaria in India. PLoS Negl Trop Dis 2016;10(2):e0005155.
10. Martens P, Hall L. Malaria on the move: human population movement and malaria transmission. Emerg Infect Dis 2000;6(2):103-109.
11. Wilson AL, Courtenay O, Kelly-Hope LA, et al. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl Trop Dis 2020;14(1):e0007831.
12. Ministry of Health and Family welfare. Entomological Surveillance Guidelines [Internet]. New Delhi: MoHFW; 2018. Available from: https://ncdc.mohfw. gov.in/showfile.php.
13. Kouassi BL, Edi C, Ouattara AF, et al. Entomological monitoring data driving decision-making for appropriate and sustainable malaria vector control in Côte d’Ivoire. Malar J 2023;22:14.
14. World Health Organization. Malaria surveillance assessment toolkit [Internet]. Geneva: WHO; 2022. Available from: http://www.who.int/publications/i/ item/9789240055278.
15. Rahi M, Das P, Sharma A. Malaria elimination in India requires additional surveillance mechanisms. J Public Health 2022;44(3):527-531.
16. Krishna C, Haradanhalli RS. Epidemiological trends of malaria in an endemic district tumkur, Karnataka. Int J Community Med Public Health 2017;4(6):2141-45.
17. Mishra AK, Bharti PK, Vishwakarma A, et al. A study of malaria vector surveillance as part of the Malaria Elimination Demonstration Project in Mandla, Madhya Pradesh. Malar J 2020;19(1):447.
18. Smith Gueye C, Newby G, Gosling RD, et al. Strategies and approaches to vector control in nine malaria-eliminating countries: A cross-case study analysis. Malar J 2016;15(1):2.
19. Gayan Dharmasiri AG, Perera AY, Harishchandra J, et al. First record of anopheles stephensi in Sri Lanka: A potential challenge for prevention of malaria reintroduction. Malar J 2017;16(1):326.
20. Dhiman R, Singh P, Yadav Y, et al. Preparedness for malaria elimination in the wake of climate change in the State of Uttarakhand (India). J Vector Borne Dis 2019;56(1):46-52.
21. Chalageri VH, Marinaik SB, Nath SN, et al. Malaria control-lessons learned from trends of Malaria indices over three decades in Karnataka, India. Malar J 2023;22:353.