RGUHS Nat. J. Pub. Heal. Sci Vol: 15 Issue: 4 eISSN: pISSN
Dear Authors,
We invite you to watch this comprehensive video guide on the process of submitting your article online. This video will provide you with step-by-step instructions to ensure a smooth and successful submission.
Thank you for your attention and cooperation.
1Dr. Manas Bajpai, Department of Oral Pathology and Microbiology, Rural Dental College, Loni, Maharashtra, India.
*Corresponding Author:
Dr. Manas Bajpai, Department of Oral Pathology and Microbiology, Rural Dental College, Loni, Maharashtra, India., Email: drmb1987@gmail.com
Abstract
Squamous cell carcinoma arising from the head and neck (HNSCC) is a challenging tumour, with high rates of recurrence and limited therapeutic options in advanced stages. The Hedgehog (Hh) signalling pathway plays a significant role in embryonic tissue development and homeostasis of tissue. Disruption of this pathway has been associated with etiopathogenesis of various cancers, including HNSCC. This paper is an attempt to explore the role of the Hh signalling pathway in HNSCC development and progression, and to critically examine the efficacy and limitations of Hh pathway inhibitors as potential therapeutic agents for these tumours. In this paper, we discuss the mechanisms and modes of action of these inhibitors, summarize clinical trial data, and discuss the limitations of their clinical application, including mechanisms of resistance and the need for combination therapies.
Keywords
Downloads
-
1FullTextPDF
Article
Introduction
Squamous cell carcinoma of the head and neck (HNSCC), encompassing cancers of the oral cavity, oropharynx, larynx, and salivary glands, represents a significant global health burden.1 Despite advancements in surgery, radiation therapy, and chemotherapy, the prognosis for patients with advanced HNSCC remains poor. This underscores the need for novel, targeted therapies to improve treatment outcomes.2
The Hedgehog (Hh) signalling pathway is a highly conserved developmental pathway crucial for cell growth, differentiation, and pattern formation. In adults, the pathway is generally quiescent but can be reactivated in certain circumstances, including tissue repair and tumorigenesis.2,3 Aberrant activation of the Hh pathway has been associated with the progression of various cancers, including basal cell carcinoma (BCC) of skin, medulloblastoma, and, increasingly, HNSCC.4 This review focused on the role of the Hh signalling pathway in HNSCC and the potential of Hh pathway inhibitors as a therapeutic strategy.
The Hedgehog Signalling Pathway: A Brief Overview
The Hh signalling pathway is initiated by the binding of a Hh ligand (Sonic Hedgehog - SHH, Indian Hedgehog - IHH, or Desert Hedgehog - DHH) to the Patched (PTCH1) receptor. With the absence of Hh ligand, PTCH1 inhibits the activity of Smoothened (SMO), a 7-transmembrane protein.2,5,6 Binding of Hh ligand to PTCH1 relieves this inhibition, allowing SMO activation.7 Activated SMO triggers a signalling cascade that ultimately activates GLI transcription factors (GLI1, GLI2, and GLI3). These GLI factors then translocate to the nucleus and regulate the expression of target genes associated with cell proliferation, survival, and differentiation.8
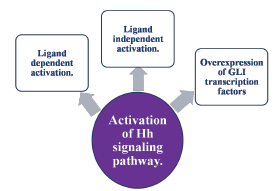
In HNSCC, the Hh signalling pathway may be activated through different mechanisms, including:
• Ligand-dependent activation: Autocrineor para-crine signalling where tumour cells themselves produce Hh ligands, leading to pathway activation.7,8
• Ligand-independent activation: Mutations in PTCH1 or SMO, resulting in combined activation of the pathway, even in the absence of Hh ligand.8
• Other mechanisms: Overexpression of GLI tran-scription factors or alterations in downstream components of the pathway (Figure 1).9
Role of the Hedgehog signalling Pathway in HNSCC
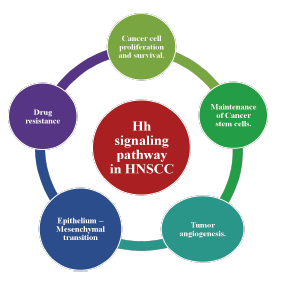
Emerging evidence reveals that the Hh signalling pathway has an extremely important role in the initiation, progression, and metastasis of HNSCC. Hh signalling promotes the proliferation of tumour cells and protects them from apoptosis. Hh pathway activation can contribute to the maintenance and regulation of stem cells of cancer (CSCs), which are believed to be the culprits for tumour initiation, recurrence, and resistance to the therapeutic modalities.2,6,10-13 Hh signalling can promote angiogenesis, the formation of new blood vessels, which is essential for tumour growth and metastasis.13 The Hh pathway is believed to induce epithelial-mesenchymal transition (EMT), a process in which epithelial cells lose their cell-cell adhesion and acquire a more migratory and invasive phenotype.14 Hh pathway activation has been linked with resistance to chemotherapy and radiation therapy in HNSCC (Figure 2).
Hedgehog Pathway Inhibitors: Mechanisms of Action and Clinical Development
Several drugs that target the Hh signalling pathway have been developed, primarily targeting the SMO protein. These are broadly classified as SMO inhibitors. Two SMO inhibitors, Vismodegib and Sonidegib, are approved by the Food and Drug Administration (FDA) for the management of basal cell carcinoma. However, their efficacy in other cancers, including HNSCC, has been less promising.15 Vismodegib (Erivedge) binds to and inhibits SMO, thereby preventing activation of downstream signalling. Sonidegib (Odomzo) also acts through the same mechanism of SMO inhibition.16
Several clinical trials have estimated the efficacy and potency of SMO inhibitors in HNSCC, both as monotherapy and in combination with other therapies. The results of these trials have been mixed, with some showing modest activity in a subset of patients, while others have shown no significant benefit.16,17
Clinical Trial Data on Hedgehog Inhibitors in HNSCC
Several clinical trials have investigated the role of Hh signalling inhibitors in HNSCC, often with the combination of standard therapies.
• Single-Agent-Studies: Early phase trials demonstrated modest clinical activity of Vismodegib and Sonidegib as monotherapy in advanced HNSCC. Responses were generally transient, and several patients developed resistance.18
• Combination Therapy Studies: Clinical trials combining SMO inhibitors with chemotherapy (e.g., platinum-based regimens), radiation therapy, or Epidermal Growth Factor Receptor (EGFR) inhibitors (e.g., cetuximab) have shown some promise, but improvements in overall survival remain limited. Key challenges include increased toxicity and development of resistance.19,20
• Biomarker-Driven Trials: Attempts to identify specific biomarkers that predict response to Hh signalling inhibitors in HNSCC have been ongoing. Although some studies have proved that increased expression of GLI1 or other Hh target genes may correlate with treatment sensitivity, definitive predictive biomarkers have not yet been identified.20
Challenges and Limitations of Hedgehog Inhibitor Therapy in HNSCC
Several factors contribute to the limited success of Hh signalling inhibitors in HNSCC.
• Mechanisms of Resistance: Resistance to SMO inhibitors is a major hurdle. Various pathways of resistance have been identified, including.20
a) Mutations in SMO: Mutations in the SMO binding pocket can prevent the drug from binding effectively.21
b) Amplification of GLI genes: Increased expression of GLI transcription factors can bypass the need for SMO activation.22
c) Activation of alternative signalling pathways: Cancer cells can activate other signalling pathways, such as the PI3K/AKT or MAPK pathways, to compensate for the inhibition of the Hh signalling pathway.20,22
• Tumour Heterogeneity: HNSCC is a highly hetero-geneous disease, with different subtypes exhibiting varying degrees of activation of Hh signalling pathway. Only a small group of tumours may be truly dependent on Hh signalling for their growth and survival.17,21,23,24
• Off-Target Effects and Toxicity: SMO inhibitors can cause various side effects, including muscle cramps, alopecia, dysgeusia (altered taste), and fatigue, which can limit their tolerability.25
• Limited Penetration of Hh Inhibitors: The tumour microenvironment in HNSCC can be dense and poorly vascularized, which may limit the penetration of Hh inhibitors into tumour cells.26
Future Directions and Potential Strategies
To improve the efficacy of Hh signalling inhibitors in HNSCC, several strategies are being explored.
• Development of Novel Hh Inhibitors: New inhibitors targeting other components of the Hh signalling pathway, including GLI inhibitors, are being developed to overcome SMO inhibitor resistance.26,27
• Combination Therapies: Combining Hh inhibitors with other targeted therapies, including EGFR inhibitors or immunotherapies, may enhance their efficacy.28
• Personalized Approach: Identifying biomarkers that predict response to Hh inhibitors will allow for a more personalized approach to treatment.29
• Targeting the Tumour Microenvironment: Combining Hh inhibitors with drugs that disrupt the tumour microenvironment may improve drug delivery and efficacy.30
• Overcoming Resistance Mechanisms: Developing strategies to prevent or overcome acquired resistance to the inhibitors of SMO is crucial. This includes agents that target the downstream GLI transcription factors, or agents that target other related signalling pathways.31,32
• Investigating the role of non-canonical Hh signalling: Emerging research suggests that Hh ligands can activate signalling pathways independent of SMO and GLI. Future studies need to clarify the role of non-canonical Hh signalling in HNSCC and its potential contribution to resistance.33
Conclusion
The Hh signalling pathway plays a complex role in the initiation and progression of HNSCC. While SMO inhibitors have shown some promise in preclinical and early clinical trials, their clinical efficacy as monotherapy has been limited by resistance mechanisms, tumour heterogeneity, and toxicity. Future research should focus on developing novel Hh inhibitors, identifying predictive biomarkers, and exploring combination therapies to overcome these limitations and improve outcomes for patients with HNSCC. A deeper understanding of the intricacies of Hh signalling in HNSCC, particularly non-canonical signalling, is essential for designing more effective targeted therapies. The future of Hh inhibitor therapy in HNSCC lies in personalized medicine, where patients are selected based on the biological characteristics of the tumour and treated with the most appropriate combination of therapies.
Supporting File
References
1. Argiris A, Karamouzis MV, Raben D, et al. Head and neck cancer. Lancet 2008;371(9625):1695-709.
2. Schmalbach CE, Miller FR. Occult primary head and neck carcinoma. Curr Oncol Rep 2007;9:(2) 139-46.
3. Varjosalo M, Taipale J. Hedgehog: Functions and mechanisms. Genes Dev 2008;22:2454-2472.
4. Yao E, Chuang PT. Hedgehog signaling: From basic research to clinical applications. J Formos Med Assoc 2015;114(7):569-76.
5. Rubin LL, de Sauvage FJ. Targeting the hedgehog pathway in cancer. Nat Rev Drug Discov 2006;5(12):1026-33.
6. Detmer K, Thompson AJ, Garner RE, et al. Hedgehog signaling and cell cycle control in differentiating erythroid progenitors. Blood Cells Mol Dis 2005;34(1):60-70.
7. Sari IN, Phi LTH, Jun N, et al. Hedgehog signaling in cancer: A prospective therapeutic target for eradicating cancer stem cells. Cells 2018;7(11):208.
8. Jin S, Liu X, Cai L, et al. Itraconazole promotes melanoma cells apoptosis via inhibiting hedgehog signaling pathway-mediated autophagy. Front Pharmacol 2025;16:1545243.
9. Persson M, Stamataki D, Welscher P, et al. Dorsal-ventral patterning of the spinal cord requires gli3 transcriptional repressor activity. Genes Dev 2002;16(22):2865-78.
10. Cong G, Zhu X, Chen XR, et al. Mechanisms and therapeutic potential of the hedgehog signaling pathway in cancer. Cell Death Discov 2025;11(1):40.
11. Bangs F, Anderson KV. Primary cilia and mammalian Hedgehog signaling. Cold Spring Harb Perspect Biol 2017;9:a028175.
12. Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol 2013;14:416- 29.
13. Ingham PW. Hedgehog signaling. Curr Top Dev Biol 2022;149:1-58.
14. Riobo-Del Galdo N, Lara Montero Á, et al. Role of hedgehog signaling in breast cancer: Pathogenesis and therapeutics. Cells 2019;8:375.
15. Bai Y, Bai Y, Dong J, et al. Hedgehog signaling in pancreatic fibrosis and cancer. Medicine 2016;95:e2996.
16. Pan Y, Zhou J, Zhang W, et al. The Sonic Hedgehog signaling pathway regulates autophagy and migration in ovarian cancer. Cancer Med 2021;10(13):4510-21.
17. Bagchi A, Bhattacharya A, Bera A, et al. PDE4 inhibitor rolipram represses hedgehog signaling via ubiquitin-mediated proteolysis of GLI transcription factors to regress breast cancer. J Biol Chem 2025;301(3):108239.
18. Humke EW, Dorn KV, Milenkovic L, et al. The output of Hedgehog signaling is controlled by the dynamic association between Suppressor of Fused and the Gli proteins. Genes Dev 2010;24:670-682.
19. Pan Y, Bai CB, Joyner AL, et al. Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol 2006;26(9):3365-3377.
20. Brennan D, Chen X, Cheng L, et al. Noncanonical Hedgehog signaling. Vitam Horm 2012;88:55-72.
21. Jing J, Wu Z, Wang J, et al. Hedgehog signaling in tissue homeostasis, cancers and targeted therapies. Sig Transduct Target Ther 2023;8(1):315.
22. Taipale J, Cooper MK, Maiti T, et al. Patched acts catalytically to suppress the activity of smoothened. Nature 2002;418(6900):892-7.
23. Azuma N, Tadokoro K, Yamada M, et al. Sonic Hedgehog determines early retinal development and adjusts eyeball architecture. Int J Mol Sci 2025;26(2):496.
24. Skoda AM, Simovic D, Karin V, et al. The role of the Hedgehog signaling pathway in cancer: A comprehensive review. Bosn J Basic Med Sci 2018;18(1):8-20.
25. Chandolia B, Rajliwal JP, Bajpai M, et al. Prognostic potential of N-Cadherin in oral squamous cell carcinoma via immunohistochemical methods. J Coll Physicians Surg Pak 2017;27(8):475-478.
26. Ballini A, Zhurakivska K, Troiano G, et al. Dietary Polyphenols against Oxidative Stress in Head and Neck Cancer: What's New, What's Next. J Cancer 2024;15(2):293-308.
27. Buglino JA, Resh MD. Hhat is a palmitoylacyltransferase with specificity for N-palmitoylation of sonic hedgehog. J Biol Chem 2008;283(32): 22076-88.
28. Martinelli DC, Fan CM. Gas1 extends the range of hedgehog action by facilitating its signaling. Genes Dev 2007;21(10):1231-43.
29. Chinchilla P, Xiao L, Kazanietz MG, et al. Hedgehog proteins activate pro-angiogenic responses in endothelial cells through non-canonical signaling pathways. Cell Cycle 2010;9:570-579.
30. Doheny D, Manore SG, Wong GL, et al. Hedgehog signaling and truncated GLI1 in cancer. Cells 2020;9(9):2114.
31. Katoh Y, Katoh M. Hedgehog signaling pathway and gastrointestinal stem cell signaling network (review). Int J Mol Med 2006;18(6):1019-23.
32. Song JH, Tieu AH, Cheng Y, et al. Novel long noncoding RNA miR205HG functions as an esophageal tumor-suppressive Hedgehog inhibitor. Cancers (Basel) 2021;13(7):1707.
33. Edeling M, Ragi G, Huang S, et al. Developmental signalling pathways in renal fibrosis: the roles of Notch, Wnt and Hedgehog. Nat Rev Nephrol 2016;12(7):426-39.